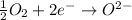Answer : The balanced chemical reaction for rusting of irons is :
![4Fe(s)+3O_2(g)+6H_2O(l)\rightarrow 2[Fe_2O_3(s).3H_2O(s)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/aubtt8thzq7rn3v8dvu7hupdqjiuznuqx3.png)
Explanation :
Iron rusting : It is a type of chemical process where an iron nail react with the water in the presence of moisture (oxygen) to give iron oxide as a product. Rusting of iron is an oxidation-reduction reaction in which iron losses electrons to oxygen atom.
Oxidation reaction : It is the reaction in which a substance looses its electrons. In this oxidation state increases.
Reduction reaction : It is the reaction in which a substance gains electrons. In this oxidation state decreases.
The balanced chemical reaction for rusting of irons is :
![4Fe(s)+3O_2(g)+6H_2O(l)\rightarrow 2[Fe_2O_3(s).3H_2O(s)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/aubtt8thzq7rn3v8dvu7hupdqjiuznuqx3.png)
Half reactions of oxidation and reduction are :
Oxidation :
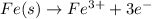
Reduction :