Answer:
Valency of the two elements
Step-by-step explanation:
In order to correctly formularize the compound, the student needs to ask of the valences of the elements involved.
Chlorine has valency of -1, while Barium +2.
The exchange of valency gives the formula
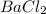 .
.
Another explanation is that Barium donates 2 electrons which the chlorine atoms accept, in order to form the covalent bond.
I hope this was helpful.