Answer: Option (c) is the correct answer.
Step-by-step explanation:
Entropy is the degree of randomness that is, more randomly the atoms of a substance are moving more will be its entropy.
For example, when ice melts then solid state of water is changing into liquid state. This, means degree of randomness is increasing.
Whereas when water is freezing then molecules are coming closer to each other. As a result, there occurs decrease in degree of randomness.
Thus, we can conclude that
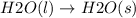 is the change that has a decrease in entropy.
is the change that has a decrease in entropy.