Answer: 7 valence electrons
Step-by-step explanation:
Electronic configuration represents the total number of electrons that a neutral element contains. We add all the superscripts to know the number of electrons in an atom.
The electrons are filled according to Afbau's rule in order of increasing energies.
Valence electrons are the number of electrons constituting the outermost shell of an atom.
Thus the electronic configuration could be :
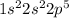
Thus the outermost orbit is 2 which has (2+5)= 7 electrons. Thus there are 7 valence electrons.