Answer:
Concentration of Ca(OH)₂:
0.117 M.
Step-by-step explanation:
How many moles of HCl is consumed?
Note the unit of concentration: moles per liter solution.
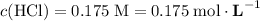 .
.
Convert milliliters to liters.
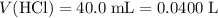 .
.
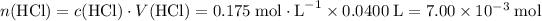 .
.
How many moles of NaOH in the solution?
Refer to the equation. The coefficient in front of Ca(OH)₂ is 1. The coefficient in front of HCl is 2. In other words, it takes two moles of HCl to neutralize one mole of Ca(OH)₂. That
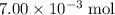 of HCl will neutralize only half that much Ca(OH)₂.
of HCl will neutralize only half that much Ca(OH)₂.
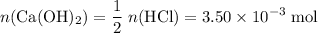 .
.
What's the concentration of the Ca(OH)₂ solution?
Concentration is the number of moles of solute per unit volume.
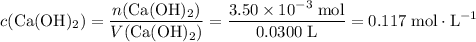 .
.