Given :
Volume of NaCl solution 2.5 L .
Molarity of NaCl solution is 0.070 M .
To Find :
How many moles are present in the solution.
Solution :
Let, n be the number of moles.
We know, molarity is given by :
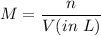
So,
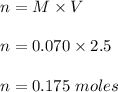
Therefore, number of moles of NaCl is 0.175 moles.