Answer:
2.7 moles of Fe₂O₃ is the maximum amount that can be produced. Iron is the limiting reactant.
Step-by-step explanation:
The balanced reaction is:
4 Fe + 3 O₂ → 2 Fe₂O₃
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of each compound participate in the reaction:
- Fe: 4 moles
- O₂: 3 moles
- Fe₂O3: 2 moles
The limiting reagent is one that is consumed first in its entirety, determining the amount of product in the reaction. When the limiting reagent is finished, the chemical reaction will stop.
You can use a simple rule of three as follows: if by stoichiometry 4 moles of Fe reacts with 3 moles of O₂, how much moles of Fe will be needed if 4.7 moles of O₂ react?
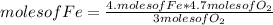
moles of O₂= 6.27
But 6.27 moles of Fe are not available, 5.4 moles are available. Since you have less moles than you need to react with 4.7 moles of O₂, iron Fe will be the limiting reagent.
So you can use a simple rule of three as follows: if by stoichiometry 4 moles of Fe produce 2 moles of Fe₂O₃, how many moles of Fe₂O₃ will be produced if 5.4 moles of Fe react?
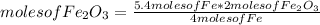
moles of Fe₂O₃= 2.7 moles
Then:
2.7 moles of Fe₂O₃ is the maximum amount that can be produced. Iron is the limiting reactant.