Answer: V₂ = 37.71mL
Step-by-step explanation: To determine the new volume of Helium gas, use the Combined Gas Law, which states the following relationship among pressure, volume and temperature:
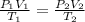
where index 1 relates to the initial state of the gas and index 2 to the final state of the gas.
Temperature is in Kelvin, so:
T = °C + 273
For this situation, standard pressure is 1 atm. Temperatures will be:
T₁ = 20 + 273 = 293 K
T₂ = 91 + 273 = 364 K
Solving:
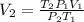
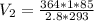
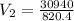
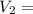 37.71
37.71
The new volume of He gas is 37.71 mL.