Step-by-step explanation:
A reaction that involves gain or loss of electrons between two atoms that chemically combine together is known as a chemical reaction.
For example,
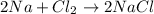 , is a chemical reaction.
, is a chemical reaction.
On the other hand, a reaction that involves combining or splitting of atomic nuclei is known as a nuclear reaction.
For example,
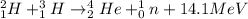
Both chemical and nuclear reactions are balanced reactions as number of atoms on reactant and product side are same. Also, both of them involve exchange of energies.
Thus, we can conclude that following statements are the same for both nuclear reactions and chemical reactions.
- Both can be represented by balanced equations.
- Both involve a change in energy.