Answer:
Concentration of OH⁻:
1.0 × 10⁻⁹ M.
Step-by-step explanation:
The following equilibrium goes on in aqueous solutions:
 .
.
The equilibrium constant for this reaction is called the self-ionization constant of water:
![K_w = [\text{H}^(+)]\cdot[\text{OH}^(-)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/hy5c3lmzh7vn9vp5r87k0gamnonky030fy.png) .
.
Note that water isn't part of this constant.
The value of
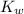 at 25 °C is
at 25 °C is
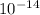 . How to memorize this value?
. How to memorize this value?
- The pH of pure water at 25 °C is 7.
![[\text{H}^(+)] = 10^{-\text{pH}} = 10^(-7)\;\text{mol}\cdot\text{dm}^(-3)](https://img.qammunity.org/2020/formulas/chemistry/high-school/g0in1igufz950km1lz3ajby4rxiey1lrf6.png)
- However,
![[\text{OH}^(-)] = [\text{H}^(+)]=10^(-7)\;\text{mol}\cdot\text{dm}^(-3)](https://img.qammunity.org/2020/formulas/chemistry/high-school/x1sq5wbshkr2qvlq3rqsnwmilvyih6cxxk.png) for pure water.
for pure water. - As a result,
![K_w = [\text{H}^(+)] \cdot[\text{OH}^(-)] = (10^(-7))^(2) = 10^(-14)](https://img.qammunity.org/2020/formulas/chemistry/high-school/zsls3fd258ng2hmbbf57dje1qu2cnwpcx4.png) at 25 °C.
at 25 °C.
Back to this question.
![[\text{H}^(+)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/go1cuj0of90izmo1vzs8if0lg4zcse0acu.png) is given. 25 °C implies that
is given. 25 °C implies that
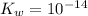 . As a result,
. As a result,
![\displaystyle [\text{OH}^(-)] = \frac{K_w}{[\text{H}^(+)]} = (10^(-14))/(1.0* 10^(-5)) = 10^(-9) \;\text{mol}\cdot\text{dm}^(-3)](https://img.qammunity.org/2020/formulas/chemistry/high-school/e1qvwr43ckzwaigt998kyvpw3w4d7iu83u.png) .
.