Answer: 80 grams
Step-by-step explanation:
This problem can be solved using the Radioactive Half Life Formula:
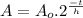 (1)
(1)
Where:
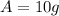 is the final amount of the material
is the final amount of the material
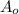 is the initial amount of the material (the quantity we are asked to find)
is the initial amount of the material (the quantity we are asked to find)
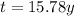 is the time elapsed
is the time elapsed
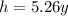 is the half life of cobalt
is the half life of cobalt
Knowing this, let's find
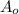 from (1):
from (1):
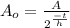
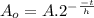
This is the same as:
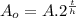 (2)
(2)
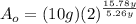
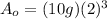
Finally:
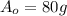 >>> This is the amount of grams in the original sample
>>> This is the amount of grams in the original sample