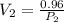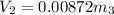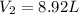Answer:
Step-by-step explanation:
We have the equation for ideal gas expressed as:
PV=nRT
Being:
P = Pressure
V = Volume
n = molar number
R = Universal gas constant
T = Temperature
From the statement of the problem I infer that we are looking to change the volume and the pressure, maintaining the temperature, so I can calculate the right side of the equation with the data of the initial condition of the gas:
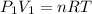
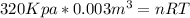
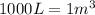
So
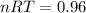
Now, as for the final condition:
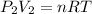
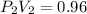
clearing