Answer: The magnetic quantum number
The magnetic quantum number
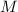 is one of the four quantum numbers that characterize the quantum state of an electron bound from an atom.
is one of the four quantum numbers that characterize the quantum state of an electron bound from an atom.
This number determines the spatial orientation of the orbital and its name is due to the fact that this spatial orientation is normally defined in relation to an external magnetic field.
Therefore:
The orientation in space of an atomic orbital is associated with the magnetic quantum number