Answer:
Photon
Step-by-step explanation:
The photon is the quantum of light. From a classifical point of view, ligth is a wave (an electromagnetic wave), but it also acts as made by particles (quanta of energy) called photons, and this behaviour is described by quantum mechanics.
When an atom changes from a higher energy level to a lower energy level, energy is released in the transition. This energy is carried away by a single photon of light. More specifically, the photon carries an energy which is equal to the difference in energy between the two levels of the atom:
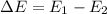
And the frequency of the photon will depend on its energy, according to the equation
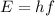
where
E is the photon's energy
h is the Planck constant
f is the photon's frequency