Answer:
Latent heat of fusion
Step-by-step explanation:
When a solid substance reaches its melting temperature, all the additional heat that is provided to the substance is used to break the bonds between the molecules to allow the change of state from solid to liquid.
During the phase change, the temperature of the substance does not change, since all the energy is used to break the bonds.
The amount of energy needed to completely melt a solid substance at its melting point is given by
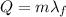
where
m is the mass of the substance
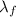 is the latent heat of fusion
is the latent heat of fusion