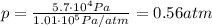Answer:
0.56 atm
Step-by-step explanation:
First of all, we need to find the number of moles of the gas.
We know that
m = 1.00 g is the mass of the gas
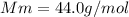 is the molar mass of the carbon dioxide
is the molar mass of the carbon dioxide
So, the number of moles of the gas is
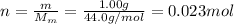
Now we can find the pressure of the gas by using the ideal gas equation:
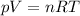
where
p is the pressure
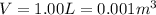 is the volume
is the volume
n = 0.023 mol is the number of moles
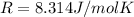 is the gas constant
is the gas constant
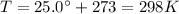 is the temperature of the gas
is the temperature of the gas
Solving the equation for p, we find
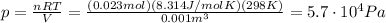
And since we have
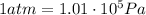
the pressure in atmospheres is