Answer:
Hexane is a saturated hydrocarbon.
Step-by-step explanation:
Saturated hydrocarbon: These are those carbon molecules which have single bond between carbon and carbon atom.
Unsaturated hydrocarbons:These are those carbon molecules which have double or triple bond between carbon and carbon atom.
Hexane is an organic molecule with molecular formula
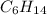 . A hydrocarbon with no double and triple bond due to which it is categorized under saturated hydrocarbon. And along with that is a non polar compound.
. A hydrocarbon with no double and triple bond due to which it is categorized under saturated hydrocarbon. And along with that is a non polar compound.