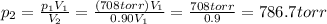Answer:
786.7 torr
Step-by-step explanation:
Boyle's law states that for a gas kept at constant temperature, the pressure and the volume are inversely proportional to each other:
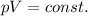
the law can be rewritten as
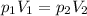
where in this problem we have:
p1 = 708 torr is the initial pressure
V1 = 899 mL is the initial volume
p2 = ? is the final pressure
V2 = 0.90 V1 is the final volume (it is 90% of the initial volume, since the volume of the container has been reduced by 10%)
So, solving the equation for p2 we find