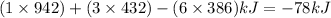Answer:
The enthalpy change for the reaction is -78 kJ
Step-by-step explanation:
In the given reaction, 1 mol of
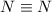 and 3 moles of H-H bonds are broken. Also 6 moles of N-H bonds are formed as 1 mol of
and 3 moles of H-H bonds are broken. Also 6 moles of N-H bonds are formed as 1 mol of
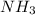 contains 3 moles of N-H bonds.
contains 3 moles of N-H bonds.
Now, energy is released during bond formation. So, for this case, sign of bond energy will be negative. Also energy if supplied during bond breaking. So, for this case, sign of bond energy will be positive.
Hence change in enthalpy of reaction=
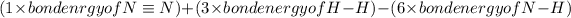 =
=