Answer: The correct answer is hydrogen gas and oxygen gas.
Step-by-step explanation:
Electrolysis of water is defined as the process in which water gets decomposed into two gases which are hydrogen and oxygen in the presence of electricity.
Hydrogen gas is released at cathode and oxygen gas is released at anode.
At anode, oxidation reaction occurs and at cathode, reduction reaction occurs.
The half reactions for the above process follows:
At anode:
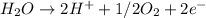
At cathode:
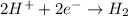
Overall reaction:
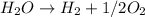
Hence, the correct answer is hydrogen gas and oxygen gas.