Answer:
At equilibrium, the forward and backward reaction rates are equal.
The forward reaction rate would decrease if
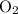 is removed from the mixture. The reason is that collisions between
is removed from the mixture. The reason is that collisions between
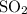 molecules and
molecules and
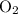 molecules would become less frequent.
molecules would become less frequent.
The reaction would not be at equilibrium for a while after
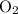 was taken out of the mixture.
was taken out of the mixture.
Step-by-step explanation:
Equilibrium
Neither the forward reaction nor the backward reaction would stop when this reversible reaction is at an equilibrium. Rather, the rate of these two reactions would become equal.
Whenever the forward reaction adds one mole of
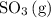 to the system, the backward reaction would have broken down the same amount of
to the system, the backward reaction would have broken down the same amount of
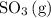 . So is the case for
. So is the case for
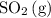 and
and
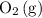 .
.
Therefore, the concentration of each species would stay the same. There would be no macroscopic change to the mixture when it is at an an equilibrium.
Collision Theory
In the collision theory, an elementary reaction between two reactants particles takes place whenever two reactant particles collide with the correct orientation and a sufficient amount of energy.
Assume that
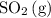 and
and
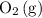 molecules are the two particles that collide in the forward reaction. Because the collision has to be sufficiently energetic to yield
molecules are the two particles that collide in the forward reaction. Because the collision has to be sufficiently energetic to yield
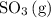 , only a fraction of the reactions will be fruitful.
, only a fraction of the reactions will be fruitful.
Assume that
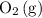 molecules were taken out while keeping the temperature of the mixture stays unchanged. The likelihood that a collision would be fruitful should stay mostly the same.
molecules were taken out while keeping the temperature of the mixture stays unchanged. The likelihood that a collision would be fruitful should stay mostly the same.
Because fewer
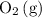 molecules would be present in the mixture, there would be fewer collisions (fruitful or not) between
molecules would be present in the mixture, there would be fewer collisions (fruitful or not) between
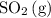 and
and
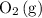 molecules in unit time. Even if the percentage of fruitful collisions stays the same, there would fewer fruitful collisions in unit time. It would thus appear that the forward reaction has become slower.
molecules in unit time. Even if the percentage of fruitful collisions stays the same, there would fewer fruitful collisions in unit time. It would thus appear that the forward reaction has become slower.
Equilibrium after Change
The backward reaction rate is likely going to stay the same right after
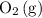 was taken out of the mixture without changing the temperature or pressure.
was taken out of the mixture without changing the temperature or pressure.
The forward and backward reaction rates used to be the same. However, right after the change, the forward reaction would become slower while the backward reaction would proceed at the same rate. Thus, the forward reaction would become slower than the backward reaction in response to the change.
Therefore, this reaction would not be at equilibrium immediately after the change.
As more and more
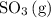 gets converted to
gets converted to
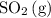 and
and
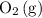 , the backward reaction would slow down while the forward reaction would pick up speed. The mixture would once again achieve equilibrium when the two reaction rates become equal again.
, the backward reaction would slow down while the forward reaction would pick up speed. The mixture would once again achieve equilibrium when the two reaction rates become equal again.