Answer:
The correct answer is option B.
Step-by-step explanation:
Mass of methanol = 32 g
Molar mass of methanol = 32 g/mol
Moles of methanol =
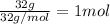
Heat of fusion of methanol =
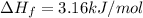
Amount energy released when 1 mol of methanol is freezes = 3.16 kJ
The correct answer is option B.