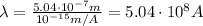Answer:
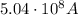
Step-by-step explanation:
The work function of the metal corresponds to the minimum energy needed to extract a photoelectron from the metal. In this case, it is:
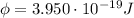
So, the energy of the incoming photon hitting on the metal must be at least equal to this value.
The energy of a photon is given by

where
h is the Planck's constant
c is the speed of light
 is the wavelength of the photon
is the wavelength of the photon
Using
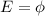 and solving for
and solving for
 , we find the maximum wavelength of the radiation that will eject electrons from the metal:
, we find the maximum wavelength of the radiation that will eject electrons from the metal:
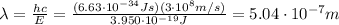
And since
1 angstrom =
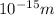
The wavelength in angstroms is