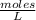Answer:
The molarity of the solution is 0.6
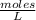
Step-by-step explanation:
Molarity (M) is the number of moles of solute that are dissolved in a given volume.
The Molarity is expressed by:
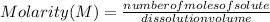
Molarity is expressed in units
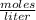 .
.
Then you must know the moles that represent 2.5 grams of LiNO₃.
For that you must know the molar mass of the compound. You know the atomic mass of the elements that make up the compound, obtained from the periodic table:
- Li: 7 g/mol
- N: 14 g/mol
- O: 16 g/mol
So, taking into account the abundance of each element in the compound:
LiNO₃= 7 g/mol + 14 g/mol + 3*(16 g/mol)= 69 g/mol
Then a rule of three applies as follows: if 69 g of LiNO₃ is 1 mol of the compound, 2.5 g of LiNO₃ how many moles are there?
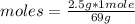
moles= 0.036
Being 60 mL = 0.06 L (1L = 1000 mL or 1 mL = 0.001 L), the molarity is calculated as:
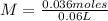
M=0.6
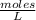
The molarity of the solution is 0.6