Answer:
[H₃O⁺] = 1.4 × 10⁻⁹ M.
Step-by-step explanation:
NH₄Cl is a salt that dissolves well in water. The 2.5 M NH₄Cl will give an initial NH₄⁺ concentration of 2.5 M.
NH₃ is a weak base. It combines with water to produce NH₄⁺ and OH⁻. The opposite process can also take place. NH₄⁺ combines with OH⁻ to produce NH₃ and H₂O. The final H₃O⁺ concentration can be found from the OH⁻ concentration. What will be the final OH⁻ concentration?
Let the increase in OH⁻ concentration be x. The initial OH⁻ concentration at room temperature is 10⁻⁷ M.
Construct a RICE table for the equilibrium between NH₃ and NH₄⁺:
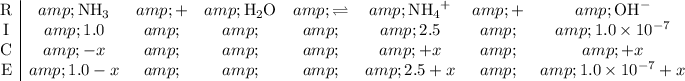 .
.
The
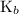 value for ammonia is small. The value of x will be so small that at equilibrium,
value for ammonia is small. The value of x will be so small that at equilibrium,
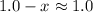 and
and
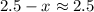 .
.
![\displaystyle \text{K}_b = \frac{[{\text{NH}_4}^(+)]\cdot [{\text{OH}}^(-)]}{[\text{NH}_3]} \approx (2.5\;(x + 1.0* 10^(-7)))/(1.0)](https://img.qammunity.org/2020/formulas/chemistry/high-school/dhibgq1jawzeqj7w2thoqkxbdan42kvj8c.png) .
.
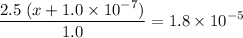 .
.
![\displaystyle [\text{OH}^(-)] = x+1.0* 10^(-7) = 1.8* 10^(-5) /\left((2.5)/(1.0)\right) = 7.2* 10^(-6)\;\text{mol}\cdot\text{L}^(-)](https://img.qammunity.org/2020/formulas/chemistry/high-school/ye2q2ngsw49ddy85c64rupinjrw9mwjt66.png) .
.
Again,
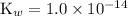 at room temperature.
at room temperature.
![\displaystyle [\text{H}_3\text{O}^(+)] = \frac{\text{K}_w}{[\text{OH}^(-)]}=(1.0* 10^(-14))/(7.2* 10^(-6)) = 1.4* 10^(-9) \;\text{mol}\cdot\text{L}^(-1)](https://img.qammunity.org/2020/formulas/chemistry/high-school/g1ssxtgefalpf8txrarcgkw09u0hnm2dii.png)