Answer:
D.The mass and the latent heat of fusion.
Step-by-step explanation:
As per the definition of latent heat of fusion we know that it is defined as the heat required to melt a given substance completely when the given substance is at its melting point point.
So here we can say that latent heat of fusion is given as
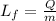
now if the heat is required to melt the given substance when it is at its melting point then it is given as
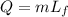
so here correct answer is given as
D.The mass and the latent heat of fusion.