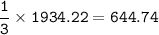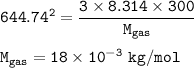The molar mass of the gas : 18 x 10⁻³ kg/mol
Further explanation
Given
An unknown gas has one third the root mean square speed of H2 at 300 K
Required
the molar mass of the gas
Solution
Average velocities of gases can be expressed as root-mean-square (V rms)
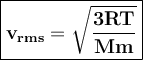
T = temperature, Mm = molar mass of the gas particles , kg/mol
R = gas constant 8,314 J / mol K
v rms An unknown gas = 1/3 v rms H₂
v rms H₂ :
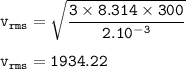
V rms of unknown gas =