Answer: b. One atom transferring electrons to another atom
Explanation: An ionic bond is formed when an element completely transfers its valence electron to another element. The element which donates the electron is known as electropositive element and the element which accepts the electrons is known as electronegative element. This bond is formed between a metal and an non-metal.
Covalent bonds are formed by sharing of electrons between non metals
For example, In calcium iodide the one electron from calcium metal gets transferred to iodine atom and thus form an ionic bond to give
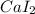
Electronic configuration of calcium:
![[Ca]=1s^22s^22p^63s^23p^64s^2](https://img.qammunity.org/2020/formulas/chemistry/high-school/lvvm0c58rxn9wtk54m0yj63em436j9pg4q.png)
Calcium atom will lose two electron to gain noble gas configuration and form calcium cation with +2 charge.
![[Ca^(2+)]=1s^22s^22p^63s^23p^6](https://img.qammunity.org/2020/formulas/chemistry/high-school/tgh352vz6ni0q9vkz0gegdru37ez3j6ors.png)
Electronic configuration of iodine:
![[I]=1s^22s^22p^63s^23p^64s^23d^(10)4p^5](https://img.qammunity.org/2020/formulas/chemistry/high-school/ym45nfe6yg01cfbvr3hmxmfw4ggxbmbynm.png)
Iodine atom will gain one electron to gain noble gas configuration and form iodide ion with -1 charge.
![[I^-]=1s^22s^22p^63s^23p^64s^23d^(10)4p^6](https://img.qammunity.org/2020/formulas/chemistry/high-school/qyx5t1t7nidcebig90x9th5wd28il80myq.png)