Answer:
2.30 × 10⁻⁸ N if the two electrons are in a vacuum.
Step-by-step explanation:
The Coulomb's Law gives the size of the electrostatic force
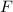 between two charged objects:
between two charged objects:
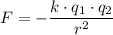 ,
,
where
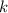 is coulomb's constant.
is coulomb's constant.
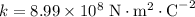 in vacuum.
in vacuum.
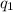 and
and
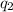 are the signed charge of the objects.
are the signed charge of the objects.
 is the distance between the two objects.
is the distance between the two objects.
For the two electrons:
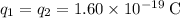 .
.
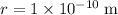 .
.
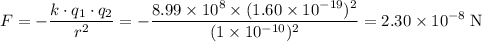 .
.
The sign of
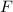 is negative. In other words, the two electrons repel each other since the signs of their charges are the same.
is negative. In other words, the two electrons repel each other since the signs of their charges are the same.