Answer:
25.6mL NaOH
Step-by-step explanation:
We are given the Molarity of the solution (
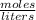 ) and the volume of the solution (.02L).
) and the volume of the solution (.02L).
By multiplying the two together, we can find the moles of solution that are reacted with HCl.
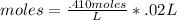
This gives us .0082 moles of HCl.
We then find the moles of NaOH that are needed to react with the HCl using the equation.
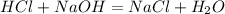
As HCl and NaCl have a 1:1 ratio, we need .0082 mol of NaOH.
Dividing this value by the Molarity of the solution
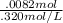
Gives us the answer, in Liters (.0256), which we can then divide by 100 convert to mL.