Answer:

Step-by-step explanation:
Hello!
In this case, since corrosion is a reaction by which a metal is oxidized to a form in which it has a higher oxidation state due to the fact that it loses electrons and therefore it becomes positively charged. In such a way, for a general metal M, we could have the following corrosion reaction:

The amount of electrons and the resulting oxidation state depend upon the nature of the metal, for instance, we can see it for silver, zinc and aluminum:
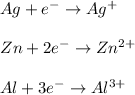
Best regards!