Answer: Option (c) is the correct answer.
Step-by-step explanation:
In an oxidation reaction there is loss of electrons whereas in a reduction reaction there is gain of electrons.
So, overall there is exchange of electrons in an oxidation-reduction reaction.
For example,
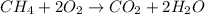 is an oxidation-reduction reaction.
is an oxidation-reduction reaction.
As there is change in oxidation number of oxygen from 0 to -2 and change in oxidation number of carbon from -4 to +4.
Thus, we can conclude that a reaction in which oxidation numbers change identifies an oxidation-reduction reaction.