Answer:
648 grams of epsom salt will be needed.
Step-by-step explanation:
Mass of magnesium atom = 24 g/mol
Mass of sulfur atom= 32 g/mol
Mass of oxygen atom= 16 g/mol
Molar mass of epsom salt:
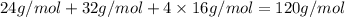
Moles of epsom salt in water = 5.40 mol
Mass of 5.40 moles of epsom salt : Molar mass × Moles:
=
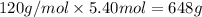
648 grams of epsom salt will be needed.