Answer:
Final pH: 9.49.
Round to two decimal places as in the question: 9.5.
Step-by-step explanation:
The conjugate of B is a cation that contains one more proton than B. The conjugate of B is an acid. As a result, B is a weak base.
What's the pKb of base B?
Consider the Henderson-Hasselbalch equation for buffers of a weak base and its conjugate acid ion.
![\displaystyle \text{pOH} = \text{pK}_b + \log{\frac{[\text{Salt}]}{[\text{Base}]}}](https://img.qammunity.org/2020/formulas/chemistry/middle-school/c620murbiry3sbr6evyruygndpve99ylj9.png) .
.
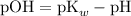 .
.
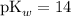 .
.
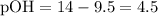
![\displaystyle \text{pK}_b = \text{pOH} -\log{\frac{[\text{Salt}]}{[\text{Base}]}}\\\phantom{\text{pK}_b} = 4.5 - \log{(0.750)/(1.05)} \\\phantom{\text{pK}_b} =4.64613](https://img.qammunity.org/2020/formulas/chemistry/middle-school/ut75o6zcdur91na8eaf1orpcoavnc1rn6i.png) .
.
What's the new salt-to-base ratio?
The 0.005 mol of HCl will convert 0.005 mol of base B to its conjugate acid ion BH⁺.
Initial:
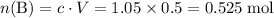 ;
;
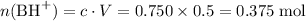 .
.
After adding the HCl:
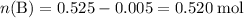 ;
;
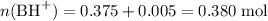 .
.
Assume that the volume is still 0.5 L:
![\displaystyle [\text{B}] = (n)/(V) = (0.520)/(0.5) = 1.04\;\text{mol}\cdot\text{dm}^(-3)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/4347ykdibp10a9czkkxarkthn3avsmr8kt.png) .
.
![\displaystyle [\text{BH}^(+)] = (n)/(V) = (0.380)/(0.5) = 0.760\;\text{mol}\cdot\text{dm}^(-3)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/eb9tjq8kn3hu70hij632stvfmqcfinu5bs.png) .
.
What's will be the pH of the solution?
Apply the Henderson-Hasselbalch equation again:
![\displaystyle \text{pOH} = \text{pK}_b + \log{\frac{[\text{Salt}]}{[\text{Base}]}} = 4.64613 + \log{(0.760)/(1.04)} = 4.50991](https://img.qammunity.org/2020/formulas/chemistry/middle-school/oeio6h0tvojvzcxev6itqhhbv2xbtmz4ju.png)
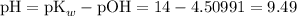 .
.
The final pH is slightly smaller than the initial pH. That's expected due to the hydrochloric acid. However, the change is small due to the nature of buffer solutions: adding a small amount of acid or base won't significantly impact the pH of the solution.