Answer:
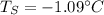
Step-by-step explanation:
Hello!
In this case, since the freezing point depression for a solution is computed via:
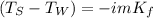
Whereas TW is the freezing temperature of water, TS that of the solution, i the van't Hoff's factor (3 for K2S as it ionizes properly), m the molality of the solution and Kf the freezing point constant of water. Thus, we plug in to obtain:
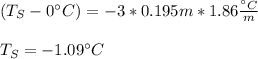
Best regards!