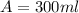Answer:
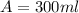
Explanation:
From the question we are told that
5% solution of dextrose sugar in water
20% solution of dextrose in
100 milliliters of distilled water water
Generally in mathematically solving we call A the amount of D20W added
Since 20% of dextrose is needed
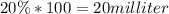
Total quantity after addition (100+x)
for DW5% total solution is
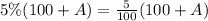
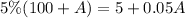
Mathematically
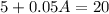
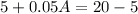
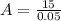
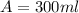
Therefore the required quantity