Answer:
(3) both particles and waves
Step-by-step explanation:
When electrons are moving with certain speed then as per particle nature of electrons we know that when electrons collide to any surface or any electron then it will transfer its momentum to other surface.
Also during collision it will follow the rules of reflection i.e. the angle made with the normal before and after collision must be same.
All these characteristics will verify the particle nature of electrons.
Now as per de-broglie hypothesis the wavelength associated with moving electron is given by
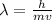
so here its wave characteristics are also represented by the diffraction of moving electrons and the interference of electrons.
So here correct answer would be
(3) both particles and waves