Answer:
the energy difference between adjacent levels decreases as the quantum number increases
Step-by-step explanation:
The energy levels of the hydrogen atom are given by the following formula:
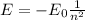
where
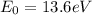 is a constant
is a constant
n is the level number
We can write therefore the energy difference between adjacent levels as
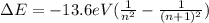
We see that this difference decreases as the level number (n) increases. For example, the difference between the levels n=1 and n=2 is
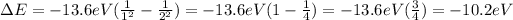
While the difference between the levels n=2 and n=3 is
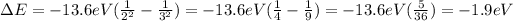
And so on.
So, the energy difference between adjacent levels decreases as the quantum number increases.