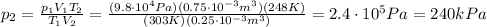Answer:
240 kPa
Step-by-step explanation:
The ideal gas law states:
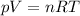
where
p is the gas pressure
V is the gas volume
n is the number of moles
R is the gas constant
T is the absolute temperature of the gas
For a fixed amount of gas, n and R are constant, so we can rewrite the equation as
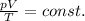
For the gas in the problem, which undergoes a transformation, this can be rewritten as

where we have:
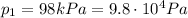 is the initial pressure
is the initial pressure
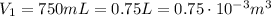 is the initial volume
is the initial volume
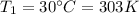 is the initial temperature
is the initial temperature
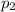 is the final pressure
is the final pressure
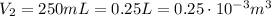 is the final volume
is the final volume
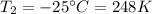 is the final temperature
is the final temperature
Solving the formula for p2, we find the final pressure of the gas: