Answer:
150.8 J
Step-by-step explanation:
The heat released by the copper wire is given by:
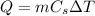
where:
m = 10.0 g is the mass of the wire
Cs = 0.377 j/(g.C) is the specific heat capacity of copper
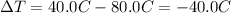 is the change in temperature of the wire
is the change in temperature of the wire
Substituting into the equation, we find
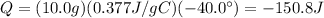
And the sign is negative because the heat is released by the wire.