Step-by-step explanation:
Molarity is defined as the number of moles divided by volume in liter.
Mathematically, Molarity =
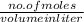
But for two solutions or mixtures with equal number of moles the formula to calculate molarity will be as follows.
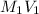 =
=
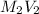
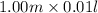 =
=
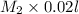
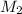 = 0.5 m
= 0.5 m
Thus, we can conclude that the molarity of the NaOH is 0.5 m.