Answer: The correct answer is Option A.
Step-by-step explanation:
Sodium is the 11th element of the periodic table having electronic configuration of
![[Ne]3s^1](https://img.qammunity.org/2020/formulas/chemistry/high-school/wfm0xsh6s3dkxk5blgxjomh7cl0mn0ln0e.png)
This element will loose 1 electron in order to attain stability and will form
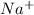 ion.
ion.
Chlorine is the 17th element of the periodic table having electronic configuration of
![[Ne]3s^23p^5](https://img.qammunity.org/2020/formulas/chemistry/high-school/e963fj2108sjpbrkolztmtjln5am3lxli2.png)
This element will gain 1 electron in order to attain stability and will form
 ion.
ion.
These two elements form ionic compound by transferring of electrons from sodium atom to chlorine atom.
Hence, the correct answer is Option A.