Answer : The number of valence electron present in an element is, 1
Explanation :
Electronic configuration : It is defined as the representation of electrons around the nucleus of an atom.
Number of electrons in an atom are determined by the electronic configuration.
Atomic number : It is defined as the number of electrons or number of protons present in a neutral atom.
The given electronic configuration of an element is,
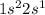
That means, the element has 1 valence electrons.