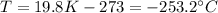Answer:
Step-by-step explanation:
First of all, we need to convert the pressure of the gas from torr to Pa. We know that:
1 torr = 133.3 Pa
So, the pressure in Pascals is
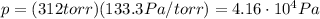
Then we also have:
n = 0.133 number of moles of the gas
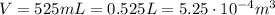 volume of the gas
volume of the gas
The ideal gas equation states that
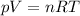
where R is the gas constant and T the absolute temperature. Solving the equation for T, we find
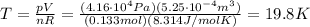
In Celsius, it becomes