Hello!
The answer is:
D.
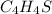
Why?
The empirical formula of a compound is the simplest formula of the same compound, if we are given the molecular formula, we can calculate the empirical formula by finding the mole ratio of each element of the compound.
So, if we know the empirical formula we will be able to get the molecular formula by multiplying it by the mole ratio.
Then, which of the options can give as a result the given molecular formula?
The answer will be D.
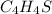 because if we multiply it by 2(mole ratio), it will give us as a result :
because if we multiply it by 2(mole ratio), it will give us as a result :
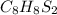
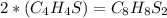
Have a nice day!