Answer: The number written below element's symbol represents the atomic number of that element
Step-by-step explanation:
The general isotopic representation of an atom is:
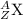
where,
Z = Atomic number of the atom
A = Mass number of the atom
X = Symbol of the atom
For Example: Isotope of chlorine (Cl-37)
Isotopic representation is
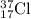
where,
17 is the atomic number of chlorine
37 is the mass number of chlorine
Cl is the symbol of chlorine element
Hence, the number written below element's symbol represents the atomic number of that element