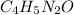Step One - multiply the molecular weight by the percent composition:
carbon: 194.19 x 0.4948 = 96.0852
hydrogen: 194.19 x 0.0519 = 10.07846
oxygen: 194.19 x 0.1648 = 32.0025
nitrogen: 194.19 x 0.2885 = 56.0238
Step Two - divide each answer by the atomic weight:
carbon: 96.0852 ÷ 12.011 = 7.9997
hydrogen: 10.07846 ÷ 1.008 = 9.998
oxygen: 32.0025 ÷ 15.9994 = 2.000
nitrogen: 56.0238 ÷ 14.0067 = 3.9997
Step Three - round off to closest whole number ratio
carbon: 8
hydrogen: 10
oxygen: 2
nitrogen: 4
The molecular formula is
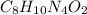
Step Four - remove common factor to get the empirical formula.
The common factor between 8, 10, 4 and 2 is 2. The empirical formula is