Answer:
The pressure of the gas will be increased to 450 kilo pascals.
Step-by-step explanation:
Since the temperature is kept constant, the volume and pressure will be related by Boyle's Law.
According to the Boyle's Law, the product of pressure and volume of a gas remains constant provided that the temperature remains constant.
Mathematically this cab be expressed as:
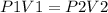
Using the given values,
P1 = 300 kilo pascals
V1 =75 L
P2 = ?
V2 = 50 L
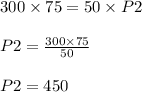
This means the pressure of the gas will be increased to 450 kilo pascals.