Answer : The correct option is, (A) Pressure is directly proportional to density.
Explanation :
Density : It is defined as the mass contained per unit volume.
Formula used for density :

From this we conclude that, the density depends on mass and volume of substance.
The density is directly proportional to the mass of substance and inversely proportional to the volume of substance.
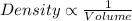
And as we know that, the volume is also depends on pressure and temperature. That means,
Volume is directly proportional to the temperature and inversely proportional to the pressure.
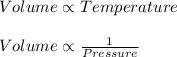
From this we conclude that,
The relation between the density, volume and pressure is:
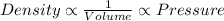
The relation between the density, volume and temperature is:
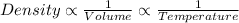
From the given option, only option A is correct option. While the other options are incorrect.
Hence, the correct option is A.