Answer: Option (c) is the correct answer.
Step-by-step explanation:
Equilibrium reaction means when rate of forward reaction is equal to rate of backward reaction.
And, relationship between the amount of products and the amount of reactants present in an equilibrium reaction at a given temperature is known as equilibrium constant.
Equilibrium constant is denoted by 'K'.
For example,
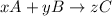
The equilibrium constant for this reaction will be as follows.
K =
![([C]^(z))/([A]^(x)[B]^(y))](https://img.qammunity.org/2020/formulas/chemistry/middle-school/kwf9zpv1ormb6zdtth1fxw6ecj2k810ocu.png)
Therefore, it is the ratio of products to reactants. And, if value of K > 1 then it means that the mixture contains mostly products whereas K < 1 means that the mixture contains mostly reactants.
Hence, we can conclude that out of the given options it is represented by the symbol K, is true for the equilibrium constant of a reaction.